Macrobrachium rosenbergii, commonly known as the freshwater prawn, has commercial importance and export potential in Bangladesh and worldwide as well. M. rosenbergii plays a crucial role in earning foreign currency for the country (Barua and Das, 2018 ![]() ). Global freshwater prawn production, dominated by M. rosenbergii, reached approximately 500,000 tons in 2016 according to FAO, (2016
). Global freshwater prawn production, dominated by M. rosenbergii, reached approximately 500,000 tons in 2016 according to FAO, (2016 ![]() ). In contrast, Bangladesh's shrimp and prawn aquaculture yielded 287.5 thousand metric tons in 2022, as reported by the Department of Fisheries (Azad and Azad, 2022
). In contrast, Bangladesh's shrimp and prawn aquaculture yielded 287.5 thousand metric tons in 2022, as reported by the Department of Fisheries (Azad and Azad, 2022 ![]() ). The success of prawn aquaculture depends on various factors, including stocking density, which affects the growth performance and overall productivity of cultivated prawns (López‐Huerta et al., 2021
). The success of prawn aquaculture depends on various factors, including stocking density, which affects the growth performance and overall productivity of cultivated prawns (López‐Huerta et al., 2021 ![]() ). Biofloc technology (BFT) is considered a promising approach in aquaculture because it provides sustainable and environmentally conscious solutions for optimal production and water quality (Ogello et al., 2021
). Biofloc technology (BFT) is considered a promising approach in aquaculture because it provides sustainable and environmentally conscious solutions for optimal production and water quality (Ogello et al., 2021 ![]() ). BFTs use beneficial microbial organisms such as bacteria, algae, and other microorganisms to convert organic waste into bioflocs, which serve as good sources of feed for cultured fish (Mugwanya et al., 2021
). BFTs use beneficial microbial organisms such as bacteria, algae, and other microorganisms to convert organic waste into bioflocs, which serve as good sources of feed for cultured fish (Mugwanya et al., 2021 ![]() ). Biofloc technology in aquaculture involves the cultivation of advantageous microorganisms to increase water quality and provide supplementary nutrition for aquatic animals, thereby increasing sustainability and efficiency (Crab et al., 2012
). Biofloc technology in aquaculture involves the cultivation of advantageous microorganisms to increase water quality and provide supplementary nutrition for aquatic animals, thereby increasing sustainability and efficiency (Crab et al., 2012 ![]() ; Bossier and Ekasari, 2017
; Bossier and Ekasari, 2017 ![]() ).
).
This system effectively mitigates the need for water exchange and external feed inputs, fostering environmentally conscious farming practices (Emerenciano et al., 2017 ![]() ; Mugwanya et al., 2021
; Mugwanya et al., 2021 ![]() ). Biofloc systems have shown promise in improving water quality, nutrient uptake, and growth performance in different aquatic species (Huang et al., 2022
). Biofloc systems have shown promise in improving water quality, nutrient uptake, and growth performance in different aquatic species (Huang et al., 2022 ![]() ). To maximize the benefits of biofloc technology in freshwater prawn production, it is vital to optimize stocking densities that promote growth and reduce stress (Khanjani and Sharifinia, 2022
). To maximize the benefits of biofloc technology in freshwater prawn production, it is vital to optimize stocking densities that promote growth and reduce stress (Khanjani and Sharifinia, 2022 ![]() ). Stocking density directly affects available space, feed competition, and interactions among prawns, all of which significantly influence their growth and survival rates (Ali et al., 2020
). Stocking density directly affects available space, feed competition, and interactions among prawns, all of which significantly influence their growth and survival rates (Ali et al., 2020 ![]() ). Optimizing stocking densities is crucial not only for enhancing growth performance but also for ensuring the economic viability of aquaculture operations (Ronald et al., 2014
). Optimizing stocking densities is crucial not only for enhancing growth performance but also for ensuring the economic viability of aquaculture operations (Ronald et al., 2014 ![]() ). Overcrowding can lead to increased resource competition, elevated stress levels, and increased vulnerability to diseases, ultimately reducing overall productivity and profitability in prawn farming (Nath and Haldar, 2020
). Overcrowding can lead to increased resource competition, elevated stress levels, and increased vulnerability to diseases, ultimately reducing overall productivity and profitability in prawn farming (Nath and Haldar, 2020 ![]() ). Conversely, understocking can result in the underutilization of available resources and infrastructure, leading to suboptimal production outcomes (Tidwell, 2012
). Conversely, understocking can result in the underutilization of available resources and infrastructure, leading to suboptimal production outcomes (Tidwell, 2012 ![]() ). Freshwater prawn cultivation usually takes place in earthen ponds via a semi-intensive method involving fertilization and supplemental feeding (New, 2005
). Freshwater prawn cultivation usually takes place in earthen ponds via a semi-intensive method involving fertilization and supplemental feeding (New, 2005 ![]() ).
).
These ponds are stocked with post-larvae or juvenile prawns at densities ranging from 4 to 20 individuals per square meter (Marques et al., 2010 ![]() ). In Bangladesh, prawn farming is performed mainly in semi-intensive systems within coastal areas (Ahmed et al., 2008
). In Bangladesh, prawn farming is performed mainly in semi-intensive systems within coastal areas (Ahmed et al., 2008 ![]() ). However, the optimal stocking densities for rearing M. rosenbergii in highly intensive biofloc systems in Bangladesh are still unknown. Therefore, determining the appropriate stocking density requires careful consideration of multiple factors, including the definite characteristics of the biofloc system, the biological needs of the prawns, and the economic goals of the aquaculture operation. Several studies have examined the impact of population densities of shrimp and freshwater prawn production in biofloc systems. These studies have provided valuable insights into the relationship between stocking density and the growth performance of these organisms. For example, Irani et al. (2023
). However, the optimal stocking densities for rearing M. rosenbergii in highly intensive biofloc systems in Bangladesh are still unknown. Therefore, determining the appropriate stocking density requires careful consideration of multiple factors, including the definite characteristics of the biofloc system, the biological needs of the prawns, and the economic goals of the aquaculture operation. Several studies have examined the impact of population densities of shrimp and freshwater prawn production in biofloc systems. These studies have provided valuable insights into the relationship between stocking density and the growth performance of these organisms. For example, Irani et al. (2023 ![]() ) reported that moderate stocking density (40 prawn/m2) had the best growth rate and survival for M. rosenbergii in biofloc systems. In parallel, Hwihy et al. (2021
) reported that moderate stocking density (40 prawn/m2) had the best growth rate and survival for M. rosenbergii in biofloc systems. In parallel, Hwihy et al. (2021 ![]() ) reported that higher stocking densities led to decreased growth rates and increased stress levels for Litopenaeus vannamei in biofloc systems. Negrini et al. (2017
) reported that higher stocking densities led to decreased growth rates and increased stress levels for Litopenaeus vannamei in biofloc systems. Negrini et al. (2017 ![]() ) reported that optimizing stocking densities is crucial for achieving optimal growth performance and maintaining the health of Penaeus monodon in biofloc systems.
) reported that optimizing stocking densities is crucial for achieving optimal growth performance and maintaining the health of Penaeus monodon in biofloc systems.
Despite the knowledge generated by these studies, the impact of stocking density on the growth performance of freshwater prawns in biofloc systems has not been studied in Bangladesh. Understanding the optimal stocking densities for M. rosenbergii in a biofloc environment can help farmers and aquaculture professionals make informed decisions to increase the productivity and sustainability of their operations. To fill this knowledge gap, this study aimed to assess the effects of different stocking densities on the growth parameters of M. rosenbergii in a biofloc system without water exchange along with water quality and plankton density.
2. Materials and Methods
2.1 Ethical approval
No ethical approval is required for this study.
2.2 Experimental site and duration
The experiment was conducted at the fish wet laboratory of the Rural Development Academy (RDA), Bogura, Bangladesh, from January 2021 to July 2021 (Figure 1).

2.3 Experimental conditions
Nine rectangular aquaria, each with a water capacity of 100 liters, were utilized for this purpose. To promote the development of natural biota, two plastic screens were attached horizontally to the bottom and vertically to the sides of each aquarium. These screens served as substrates and provided additional feed for the prawns, aided in nitrification, ensured a more uniform distribution of the prawns, and reduced their relative density (Ballester et al., 2017 ![]() ). Nets were placed over all the aquaria to prevent the prawns from escaping. Forty days prior to the inception of the experiment, a separate tank was filled with clear water. Subsequently, 50 liters of microorganism-rich water (green water) was added to the external tanks. This tank, known as the mother tank (Avnimelech, 1999
). Nets were placed over all the aquaria to prevent the prawns from escaping. Forty days prior to the inception of the experiment, a separate tank was filled with clear water. Subsequently, 50 liters of microorganism-rich water (green water) was added to the external tanks. This tank, known as the mother tank (Avnimelech, 1999 ![]() ), housed 1000 prawns, resulting in a stocking density of 230 prawns/m². The purpose of this tank was to support and facilitate the development of biofloc. Furthermore, 7.5 g of probiotics (Pondcare 35, 35 billion CFU/gram, Eskayef Pharmaceuticals LTD.) was added to the mother tanks daily. The probiotics were allowed to mature for eight hours before being used in the mother tank in another container with 10 L of water. Six pieces of air stone were provided in the mother’s tank. On the other hand, a single air stone was provided in each experimental aquarium from two aerators with backup for 24 hours throughout the experiment to increase the biofloculation rate.
), housed 1000 prawns, resulting in a stocking density of 230 prawns/m². The purpose of this tank was to support and facilitate the development of biofloc. Furthermore, 7.5 g of probiotics (Pondcare 35, 35 billion CFU/gram, Eskayef Pharmaceuticals LTD.) was added to the mother tanks daily. The probiotics were allowed to mature for eight hours before being used in the mother tank in another container with 10 L of water. Six pieces of air stone were provided in the mother’s tank. On the other hand, a single air stone was provided in each experimental aquarium from two aerators with backup for 24 hours throughout the experiment to increase the biofloculation rate.
2.4 Stocking densities and management system
Prawn post larvae (PLs) after 12 days were collected from the government fish production hatchery, Mithapukur, Rangpur, Bangladesh. The PLs were then reared in the mother tank for up to 40 days with no water exchanged, following Liu et al. (2017 ![]() ). Molasses was added as a carbon source in the mother tank when the total ammonia nitrogen (TAN) concentration was raised above one (1.0) ppm. For each (1.0) ppm increase in TAN in the tank, 6.0 g of carbon was added during the nursery period (40 days) (Ebeling et al., 2006
). Molasses was added as a carbon source in the mother tank when the total ammonia nitrogen (TAN) concentration was raised above one (1.0) ppm. For each (1.0) ppm increase in TAN in the tank, 6.0 g of carbon was added during the nursery period (40 days) (Ebeling et al., 2006 ![]() ). After the nursery period, prawns of similar size and weight were sorted from the mother tank to be stored in the experimental glass aquaria. The experiment was carried out under three treatments (T-1=50 prawn m-2, T-2= 70 prawn m-2 and T-3= 90 prawn m-2), with three replications for 180 days, as described by Negrini et al. (2017
). After the nursery period, prawns of similar size and weight were sorted from the mother tank to be stored in the experimental glass aquaria. The experiment was carried out under three treatments (T-1=50 prawn m-2, T-2= 70 prawn m-2 and T-3= 90 prawn m-2), with three replications for 180 days, as described by Negrini et al. (2017 ![]() ).
).
2.5 Feeding
The post-larvae of prawns were provided with a commercial diet containing 40% crude protein, which was fed three times daily. Initially, prawns were provided with food equivalent to 7% of their biomass. Feeding was offered at 8:00 am, with 30% of the daily amount given, followed by another 30% at 13:30 pm and the remainder at 17:30 pm. The daily feeding rate was adjusted to ensure complete consumption, and feed quantities were recorded to calculate feed utilization parameters. Additionally, pre-weighed molasses was added daily at 15:30 pm to maintain an optimal C/N ratio. Kaldnes biomedia (K2) media was also introduced into the nitrifying bacterial habitat to enhance the nitrification process.
2.6 Water quality monitoring
Daily measurements were taken for temperature, dissolved oxygen, and pH in each experimental unit via a multi-parameter device (model: HI 98194, HANNA, Romania). Total ammonia nitrogen (TAN) levels were assessed thrice weekly, whereas nitrite (NO2), alkalinity, and hardness were monitored weekly via the procedures outlined by Gunther et al. (1981 ![]() ). To maintain the water hardness above 20 mg/L, calcium carbonate was periodically added at a concentration of 0.5 g/L to the experimental units, as recommended by Arana (2010
). To maintain the water hardness above 20 mg/L, calcium carbonate was periodically added at a concentration of 0.5 g/L to the experimental units, as recommended by Arana (2010 ![]() ).
).
2.7 Determination of growth performance
After the experiment, we counted the remaining prawns in each experimental unit to determine the survival rate. Additionally, we used an analytical digital scale (accurate to 0.01 g) to measure the total individual weight of the prawns in each experimental unit. This measurement was then used to calculate the weight gain (WG), specific growth rate (SGR), and feed conversion rate (FCR).

2.8 Determination of planktonic microorganisms present in the floc
To determine the abundance of plankton, water samples were collected at the end of the experiment from each treatment and preserved in a 4% buffered formalin solution (Thompson et al., 2002 ![]() ). During each sampling, ten liters of water were taken from the experimental unit and passed through a 25 µm mesh plankton net for filtration. A dropper was used to transfer one ml of the concentrated plankton sample to the Sedgwick-Rafter (S-R) counting cell, which has a total volume of 1000 mm³ or 1 ml. The S-R cell was divided into 1000 fields, each with a volume of 0.001 ml. Before counting, the S-R cells were left undisturbed for at least 15 minutes to allow the plankton to settle. Next, the cell was placed on a camera microscope with phase contrast (Edler and Elbrächter, 2010
). During each sampling, ten liters of water were taken from the experimental unit and passed through a 25 µm mesh plankton net for filtration. A dropper was used to transfer one ml of the concentrated plankton sample to the Sedgwick-Rafter (S-R) counting cell, which has a total volume of 1000 mm³ or 1 ml. The S-R cell was divided into 1000 fields, each with a volume of 0.001 ml. Before counting, the S-R cells were left undisturbed for at least 15 minutes to allow the plankton to settle. Next, the cell was placed on a camera microscope with phase contrast (Edler and Elbrächter, 2010 ![]() ). Planktonic organisms in 10 randomly selected fields out of the 1000 fields were then counted. The abundance of plankton was subsequently calculated via the formula provided by Rahman (1992
). Planktonic organisms in 10 randomly selected fields out of the 1000 fields were then counted. The abundance of plankton was subsequently calculated via the formula provided by Rahman (1992 ![]() ).
).

N represents the number of plankton cells per liter, A denotes the total number of plankton counted, C is the volume of the final concentrate of samples in milliliters, V represents the volume of a field in cubic millimeters, F denotes the number of fields counted, and L is the volume of original water in liters.
The average plankton count was quantified as the number of cells per liter of water (cells/L). Planktonic images were captured and analyzed via IMAGEJ software, version 1.47, on the basis of the morphological characteristics of a digital microscope with a camera (Model: B-290TB, OPTIKA Italy).
2.9 Statistical analysis
The water quality data and growth parameters were subjected to repeated-measures ANOVA to assess significant differences at the 5% significance level (α=0.05). Post hoc analysis with the Tukey test (P<0.05) was conducted where significant differences were identified. Statistical analyses were carried out via IBM SPSS Statistics for Windows, version 25.0.
3. Results and Discussion
Water quality parameters
During the experimental period, all the water quality parameters except alkalinity were within the optimum range. The water quality parameters are tabulated in Table 1. Alkalinity frequently fluctuated throughout the entire experimental period, indicating a loss of buffering capacity in the biofloc systems. As a result, frequent additions of NaHCO3 are required (Azim and Little, 2008 ![]() ). In biofloc farming systems, alkalinity and pH naturally tend to decrease, whereas nitrogen compound concentrations tend to increase (Wasielesky et al., 2006
). In biofloc farming systems, alkalinity and pH naturally tend to decrease, whereas nitrogen compound concentrations tend to increase (Wasielesky et al., 2006 ![]() ). Any deviations in water quality parameters from the optimum range were addressed by supplementing necessary aqua products or increasing aeration (Ebeling et al., 2006
). Any deviations in water quality parameters from the optimum range were addressed by supplementing necessary aqua products or increasing aeration (Ebeling et al., 2006 ![]() ).
).
Table 1. Observed water quality parameters during the experiment.
3.2 Growth performance of freshwater prawns
The growth performance of freshwater prawns (M. rosenbergii) in a biofloc system was evaluated during the experimental period. The prawns in T-1 presented the greatest weight gain (35.35±8.541), although the difference was not statistically significant. In T-2 and T-3, the weight gain was 28.18±5.19 and 26.58±7.61, respectively (Table 2).
The SGR values were 2.04±0.08, 1.94±0.06, and 1.90±0.06 for the three treatments, respectively. In contrast, the lowest FCR at the end of the experiment was 1.91±0.104 in T-1, whereas T-2 and T-3 presented FCR values of 1.96±0.20 and 2.15±0.27, respectively. However, there was no statistically significant variation in weight gain, SGR, or FCR among the treatments. T-1 presented the highest survival rate (74.00±9.57), which significantly differed (P<0.05) from those of T-2 and T-3 (Table 2).
Table 2. Growth parameters in the three different treatment groups (means ± SD).
In this experiment, a lower stocking density resulted in greater growth performance. Several researchers have noted that M. rosenbergii has higher production and survival rates when it is stored at lower densities in experimental tanks (David et al., 2015 ![]() ; El-Sherif and Mervat, 2009
; El-Sherif and Mervat, 2009 ![]() ; Paul et al., 2016
; Paul et al., 2016 ![]() ). When freshwater prawns are stocked at high densities in different farming systems, they compete for food and space, leading to hierarchical competition and increased cannibalism (Moraes-Valenti et al., 2010
). When freshwater prawns are stocked at high densities in different farming systems, they compete for food and space, leading to hierarchical competition and increased cannibalism (Moraes-Valenti et al., 2010 ![]() ; Arnold et al., 2006
; Arnold et al., 2006 ![]() ; Sampaio and Valenti, 1996
; Sampaio and Valenti, 1996 ![]() ). In this study, a relatively high stocking density did not significantly affect weight gain, SGR, or FCR. However, a relatively low stocking density significantly influenced the survival rate of M. rosenbergii. Under crowded conditions, intraspecific competition among freshwater prawns prompts a strategy to reduce the population size rather than affecting the growth parameters (Begon et al., 2007
). In this study, a relatively high stocking density did not significantly affect weight gain, SGR, or FCR. However, a relatively low stocking density significantly influenced the survival rate of M. rosenbergii. Under crowded conditions, intraspecific competition among freshwater prawns prompts a strategy to reduce the population size rather than affecting the growth parameters (Begon et al., 2007 ![]() ). Furthermore, the lower density (T-1) in the present study resulted in a more efficient FCR. As the stocking density of freshwater prawns increases, the efficiency of FCR decreases (Paul et al., 2016
). Furthermore, the lower density (T-1) in the present study resulted in a more efficient FCR. As the stocking density of freshwater prawns increases, the efficiency of FCR decreases (Paul et al., 2016 ![]() ; El-Sherif and Mervat, 2009
; El-Sherif and Mervat, 2009 ![]() ), which may be attributed to cannibalistic activities and growth differences between male and female freshwater prawns (Pérez-Rostro et al., 2014
), which may be attributed to cannibalistic activities and growth differences between male and female freshwater prawns (Pérez-Rostro et al., 2014 ![]() ).
).
3.3 Total abundance of plankton present in the floc
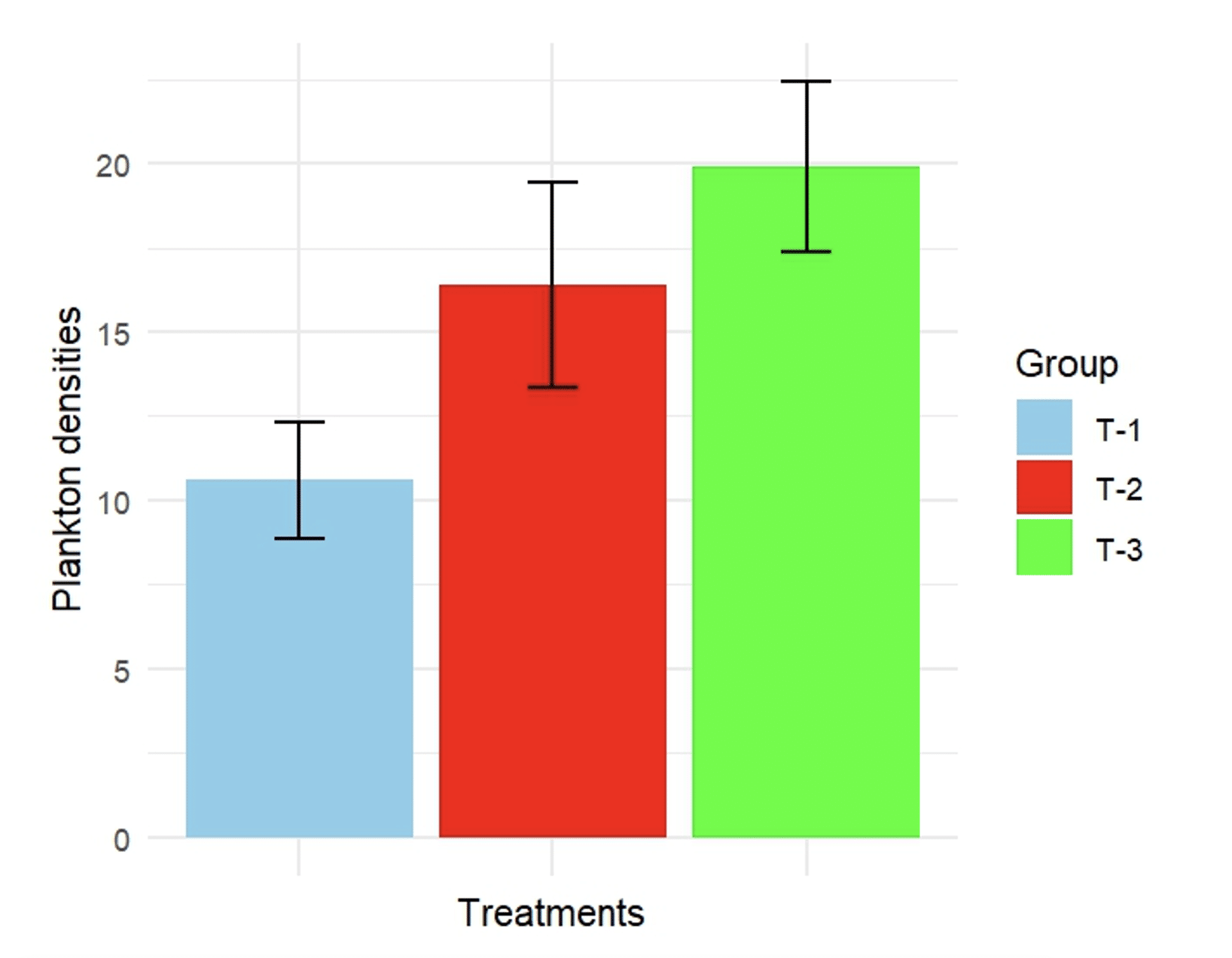
The total abundance of plankton in T-3 was 19.9×104 cells/L, which was greater than that in the other two treatments. In T-1 and T-2, the abundances of plankton were 10.61×104 and 16.4×104 cells/L, respectively (Figure 2), and the microscopic views of the plankton densities and major individual plankton are shown in Figure 3 and Figure 4, respectively.
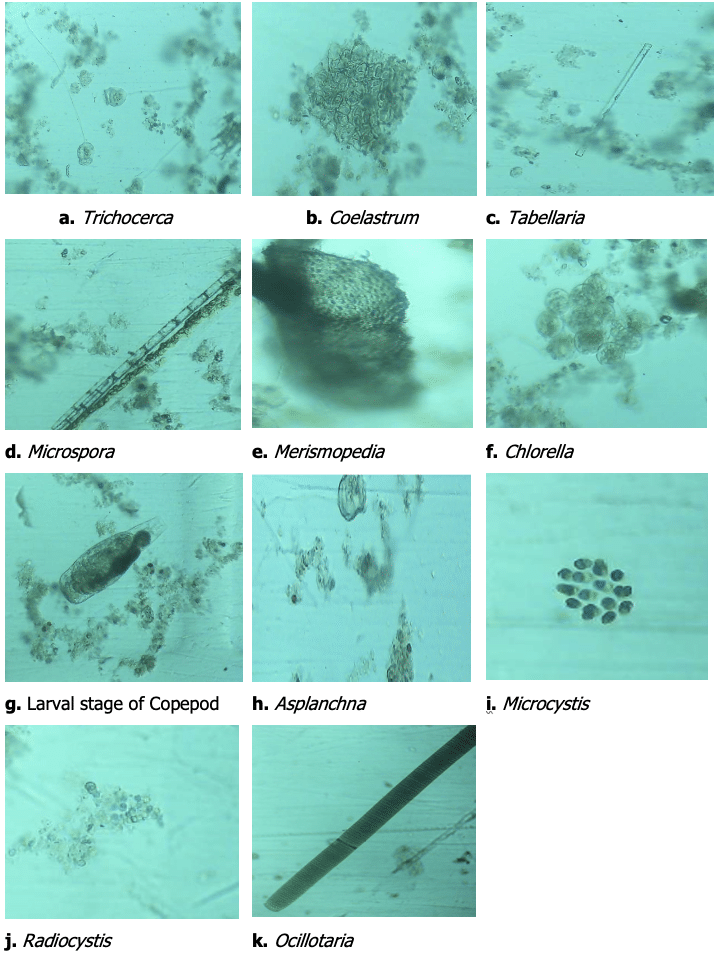
Among the three treatments, a total of eleven plankton genera were found, of which eight were phytoplankton and three were zooplankton. The eight phytoplankton genera were categorized into three major groups: Cyanophyceae, Bacillariophyceae, and Chlorophyceae. The Cyanophyceae group consisted of Microcystis, Radiocystis, Merismopedia, and Ocillotaria.

The Bacillariophyceae group consisted of one genus, Tabellaria, whereas the Chlorophyceae group consisted of Coelastrum, Microspora, and Chlorella. In this study, three genera of zooplankton were identified: Asplanchna, Trichocerca (rotifers), and Cyclops. Among the phytoplankton, the Cyanophyceae group was the most dominant, while the rotifers were the most dominant group of zooplankton.
Ekasari and Maryam (2012 ![]() ) reported that Bacillariophyceae and protozoa were the dominant groups in the biofloc system of Tilapia. Asaduzzaman et al. (2010
) reported that Bacillariophyceae and protozoa were the dominant groups in the biofloc system of Tilapia. Asaduzzaman et al. (2010 ![]() ) also reported that rotifers were the dominant group in the biofloc system of M. rosenbergii. The differences in the dominant group of plankton in the present study may be due to species differences or other factors.
) also reported that rotifers were the dominant group in the biofloc system of M. rosenbergii. The differences in the dominant group of plankton in the present study may be due to species differences or other factors.
4. Conclusions
This research has shown that the growth performance of freshwater prawns (M. rosenbergii) in a biofloc system is affected by stocking density. The main findings suggest that low stocking densities result in the best growth rates and survival rates for the prawns. These findings emphasize the importance of carefully managing stocking densities to optimize the productivity of biofloc systems. The application of these findings can assist aquaculture professionals in Bangladesh in improving prawn farming practices in biofloc systems, potentially leading to increased yields and sustainability. However, there is still a significant research gap in understanding the long-term effects of different stocking densities on prawn health and water quality parameters. Additionally, further research could explore the economic implications of adopting optimal stocking densities in commercial prawn farming operations.
Acknowledgements
We would like to express our gratitude to the Rural Development Academy (RDA), Bogura, for their generous financial support. Their commitment to advancing aquaculture research is greatly appreciated.
Data availability statement
The first author can provide the data upon reasonable request.
Informed consent statement
Not applicable.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author contributions
MAA: Conceptualization, methodology, formal analysis, investigation, data curation, writing; MAK: Validation, supervision, writing—review and editing. All the authors critically reviewed the manuscript and agreed to submit a final version of the article.




